by Greg Hunt, J Krispn Given, Jennifer M. Tsuruda, Gladys K. Andino
Introduction.
Despite the general recognition among beekeepers and bee researchers that Varroa mites are the number one risk factor for honey bee colony mortality, a look at the Bee Informed Partnership national surveys tells us that most beekeepers are hobbyists and most of them do nothing to control for Varroa mites in any given year, and those that do not control mites have much higher colony losses (2010-2014). There are some non-chemical practices that beekeepers use that help control mite levels such as introducing a break in the brood cycle by splitting colonies and re-queening, or the use of screened bottom boards. There are also some commonly used mite control practices that research has shown are ineffective, for example the use of comb with small cell sizes (Zhou et al. 2001; Taylor et, al. 2008; Ellis et al. 2009; Berry et al. 2010; Coffey et al. 2010; Seeley and Griffin 2011). One important non-chemical strategy for sustainable beekeeping is use of mite-tolerant honey bee stocks.
Progress in selecting for resistance to Varroa has been slow but there is evidence that the bees have begun their own fight against the mites. Some queen breeders are trying to help bees in this fight by incorporating lines of bees that have been subjected to natural selection by surviving without miticide treatment, such as Russian bees imported to North America by the USDA (Rinderer et al. 2010). Another approach is to select for specific traits that are effective at lowering mite populations. A study in Europe found that colonies with low mite populations had damaged mites falling from the bees (Moosbeckhofer 1992) and other studies have suggested that grooming behavior is important for resisting mite infestation, as it is in the mite’s original host, the Asian honey bee (Peng et al. 1987; Boeking and Spivak 1999; Mondragon et al. 2005). However the benefit of using the proportions of damaged mites as selection criteria has been questioned (Rosenkranz et al. 1997).
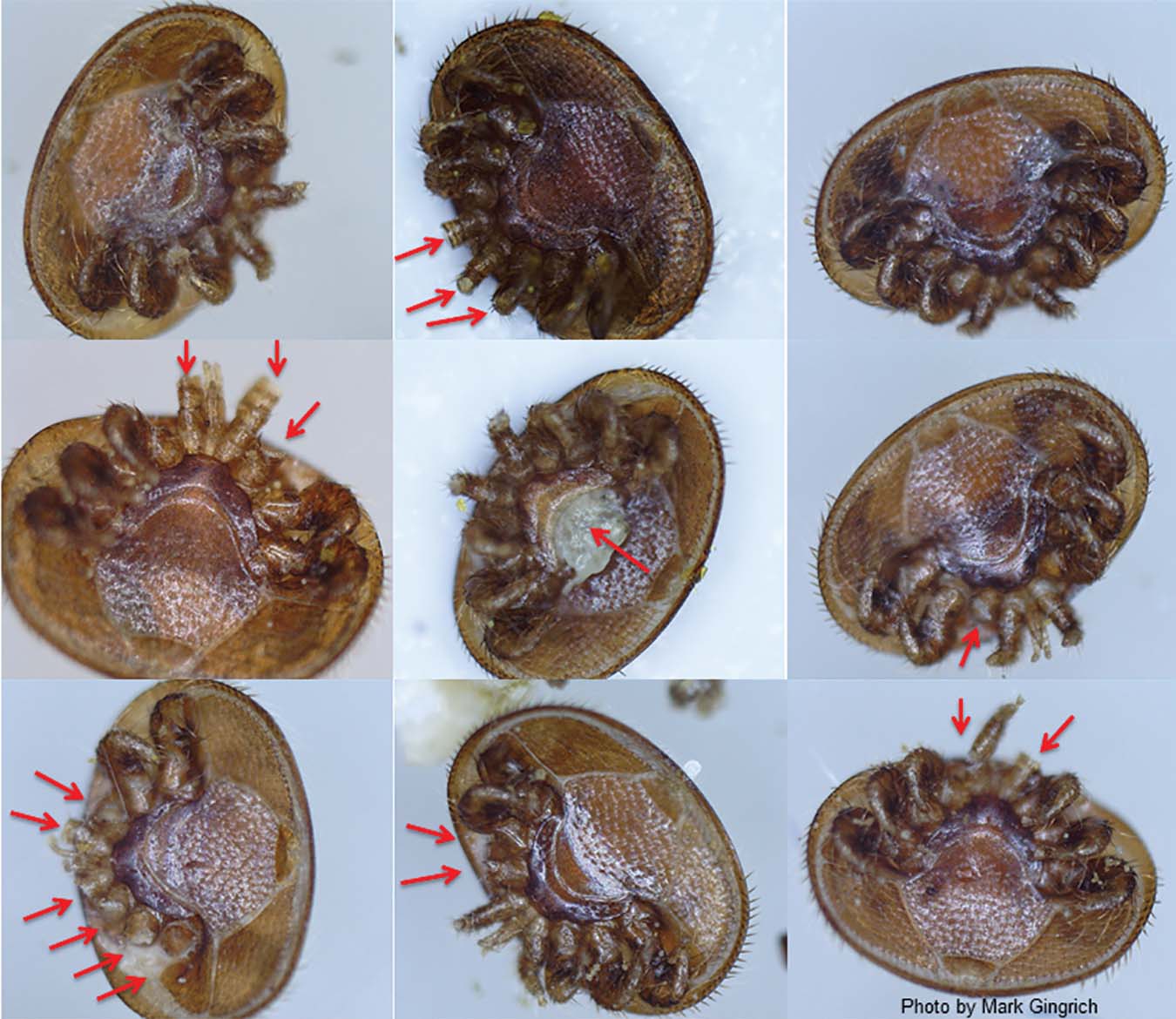
Mites that have been chewed by the mite-biters
Varroa-sensitive hygiene (VSH) was discovered as an important mite resistance mechanism by measuring the growth of Varroa populations in many colonies with queens that came from different sources (Harbo and Harris 1999; 2005). The VSH trait has been effectively incorporated into breeding lines and VSH queens are commercially available (Rinderer et al. 2010). A similar study with another set of queens showed that in those colonies grooming behavior was the trait that was most closely associated with reduced mite levels. Higher-grooming colonies also were more likely to bite the mites (Arechavaleta-Velasco and Guzmán-Novoa 2001). At least two other studies also showed a link between grooming behavior and the proportion of chewed mites falling from colonies. Cages of bees that removed a higher proportion of mites from themselves in a lab assay had fewer mites left on the bees, and the proportion of mites removed correlated with the proportion of damaged mites on sticky boards from the source colonies (Andino and Hunt 2011). Another study looked at four pairs of allegedly mite-tolerant and mite-susceptible lines from different populations. In each comparison, the more resistant stocks had more vigorous and effective individual grooming behavior when a mite was put on worker bees, and had higher proportions of chewed mites falling from the source colonies (Guzman-Novoa et al. 2012). This means that when bees are selected for low mite population growth they tend to be better groomers. Genetic studies identified regions of honey bee chromosomes and candidate genes that influence both of these complementary resistance traits but using DNA markers to select for good resistance genes is not very practical in our opinion (Tsuruda et al. 2012; Arechavaleta-Velasco et al. 2012). Even after confirming an individual gene’s effect on a trait, the value of selecting based on DNA or protein markers would be limited because other unknown genes also influence these traits so you would only increasing the frequency of some of the “good genes.” It seems that at least for now the best way forward is to select based on the trait itself. We conducted a breeding program to select for “mite biters.” Here we describe some of the selection methods, correlations between measures of grooming behavior and mite levels, and the results of a beekeeper stock evaluation.

Krispn Given looking at mites.
Methods.
Breeding population and selection. The breeding population was established in 1997 from diverse sources, including queens from commercial queen breeders: some Carniolans from California breeders and one each of VSH and Russian colonies from Glen Apiaries. But many of the colonies in the population were those that had survived for years without miticide treatments. Each year the population consisted of about 100 colonies. It was not a closed breeding population. Occasionally, queens from other Midwest queen producers or feral colonies were introduced. Queens were all marked with paint and records kept of supercedure events. Initially, breeder queens were either instrumentally inseminated or open-mated. Daughter queens were open-mated in two mating yards one mile apart and isolated from all but a few other beehives by two miles. They contained selected drone-producing colonies with one or two frames of drone comb. From 1997-2006 breeder colonies were selected based only on low mite population growth as measured by two to three counts of mite fall on sticky-board sampling sheets during the Spring and Summer.
Beginning in 2007 we began selecting for mite-grooming behavior. For the early years we treated colonies with miticide if Varroa levels were too high (usually >100 mites falling in a day late in the season). For the past six years no mite controls have been used and we do not split the colonies very often so there are minimal breaks in the brood cycle, which would reduce mite levels. Breeder queens were selected based on the proportion of mites that had damaged legs or an apparent bite in the body (the idiosoma) of the mite. To measure the proportion of chewed mites, plastic sampling sheets were sprayed with vegetable oil and slid underneath colonies that had screened bottom boards (Country Rubes, Grass Valley, CA) and left for two or three days. Using enough vegetable oil makes it fatal for the mites, and also for any ants that might try to feed on them. Mites were carefully removed from the sample board using a small paintbrush and placed belly up (ventral side) in rows on microscope slides. If fewer than 10 mites were present the data was recorded but not used for selection decisions. The number of mites on sticky boards was recorded, slides were examined with a microscope (15X), and the number of mites missing legs or leg parts or showing mutilation of the idiosoma was counted. Pale immature mites were not examined because these could have fallen as bees emerge from brood cells and may be more susceptible to damage unrelated to grooming behavior. Sometimes empty shells – the idiosoma with virtually no contents were observed. These were not counted because we do not know their cause. The relative severity of mutilations was also scored as low, moderate or high, meaning most mites had multiple legs chewed and bites to the idiosoma were seen. Selection was hierarchical, which means that we first selected colonies with the highest proportion of chewed mites that were highly mutilated. We secondarily selected for low mite population growth and colony strength over the season. Colonies were re-queened if they had high mites or had chalkbrood or other brood diseases. A hygienic behavior test was usually performed on potential breeder queen colonies, which were required to show at least 95% hygienic removal of freeze-killed brood within 24 h (Spivak and Downey 1998).
In 2009 selection was based on the results of laboratory grooming assays for mite removal as well as the proportion of chewed mites in a colony (Andino and Hunt 2011). Beginning in 2010, we tested all of the colonies at least two or three times per season for the proportion of chewed mites and each breeder queen was instrumentally inseminated with semen from multiple drones from one or two selected hives. During 2013 and 2014, we tested for correlations between mite drop and the proportion of chewed mites. Because of a mistake that was made in 2014, the total number of mites was not counted in colonies that had more than 70 mites falling on the sampling sheets but the proportion of chewed mites was recorded for a sample of 70 mites. There were seven of 56 and 19 of 63 colonies in this category for May and August measurements, respectively.
Beekeeper stock evaluation. In late June 2014 we initiated a beekeeper stock evaluation program by providing marked commercial-source queens and “IN mite biters” from the Purdue breeding program to beekeepers. This was a blind study; beekeepers received marked queens to identify the sources but were not told which ones were mite-biters. Beekeepers were asked not to treat to control Varroa mites. We purchased queens from three Western queen breeders (two Carniolan and one Italian). We chose two IN mite-biter grafting sources to test. Participants were asked to de-queen a colony and split it so that one commercial-source and one IN queen could be introduced into each half, presumably starting with equal mite loads. Beekeepers were asked not to treat colonies to control mites and to report whether they survived for a full year. Some beekeepers also provided data on mite levels, honey production, personal observations and preference for one queen source over another.
Results and Discussion.
The proportion of chewed mites is a heritable colony trait:
It was possible to increase the proportion of chewed mites in the breeding population, even though we selected from a base population of only about 100 colonies. These results show that the trait is heritable. Starting with an average of 3% chewed mites, the proportion of chewed mites increased steadily (Figure 1). There no doubt is experimental variation because different observers scored the mite damage, but only one individual scored mites in any given year. We have also observed VSH activity in some colonies in the breeding population but haven’t routinely tested for it. Because the starting average for chewed mites was low (3%), it could be that the earlier selection for low mite populations resulted in higher levels of VSH in the breeding lines.
Evidence that grooming reduces mite populations:
On the two sampling dates in 2014 we observed a significant inverse relationship between the proportion of chewed mites and mite drop, suggesting that grooming behavior is effective in reducing mite levels (Table 1). We were not sure whether to include colonies that dropped fewer than 10 mites or the colonies that had 70 or more (reported as just 70), so we analyzed the data with and without these colonies. In general, colonies with fewer mites on sticky boards had higher proportions of chewed mites. Although we always saw a trend in this direction, the correlation was not statistically significant in 2013. During 2013 there was high variation in mite levels. There were 13 out of 42 colonies in June that had less than 10 mites falling on the sampling sheet but five had 122-277 mites falling during the same three days. By August the variation in mite drop among hives was even higher. It is possible that when mite populations get too high that grooming behavior is insufficient to control population growth and the proportion of chewed mites is lower as a result. One difficulty in finding a relationship between mite-biting and mite levels in our colonies may be that they are not uniform in size and in colony history. For example, mite populations decline when a colony is re-queened because there is a break in the brood cycle and mite levels are higher in colonies that have a lot of brood. We also do not know how much VSH behavior varies in the colonies. One of our grafting sources in 2014 exhibited this trait by removing mite infested brood within 48 hours (Fig 1). It maintained low mite populations and had a high proportion of chewed mites dropping on the sticky board. But in general the 2014 results suggest that grooming is effective at reducing mite levels, at least when the mite population is not too high.
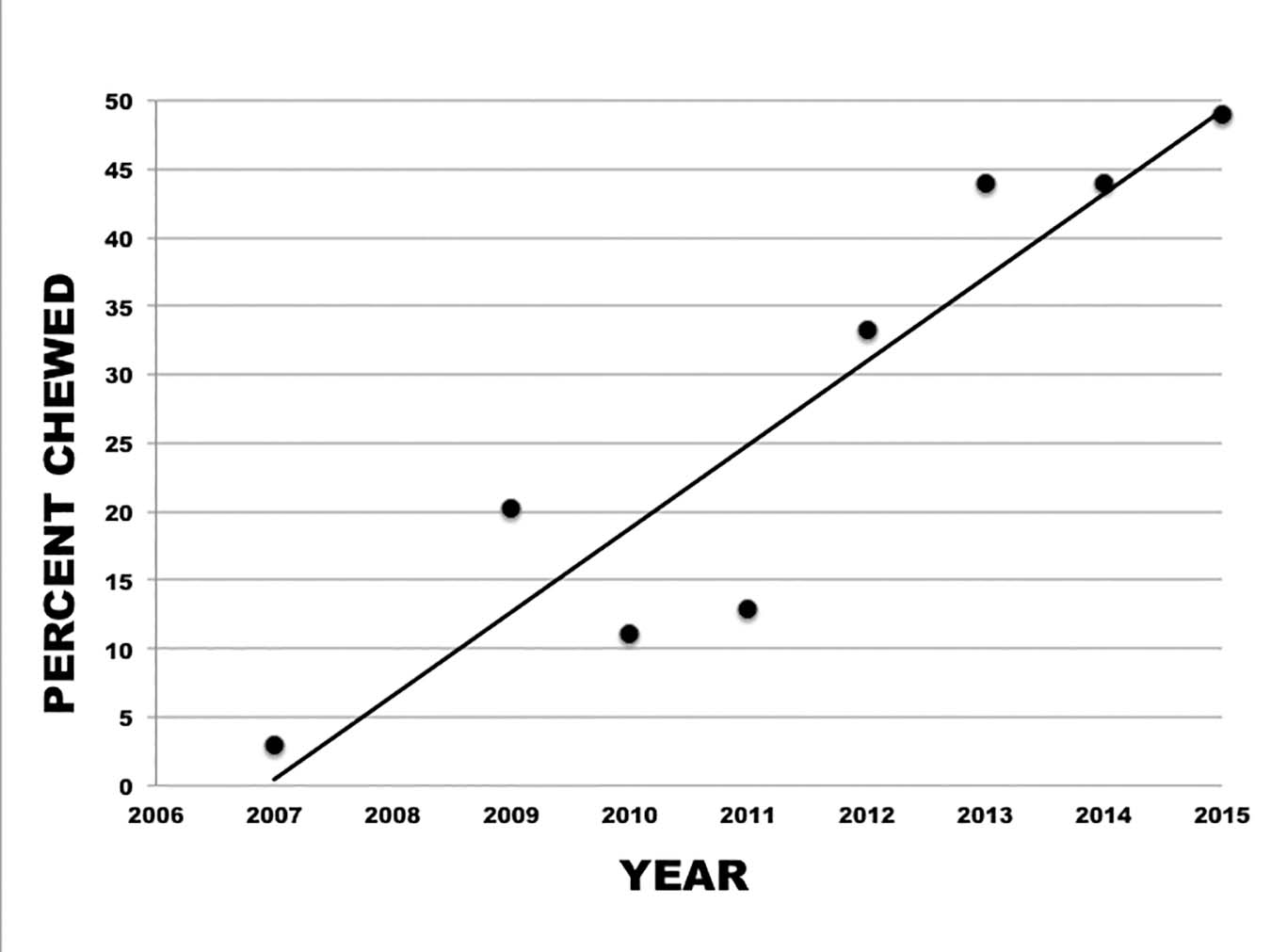
Figure 1. Response to selection for chewed mites. The average proportion of chewed mites increased steadily in the breeding population.
There appears to be good reasons for bees to bite mites. Bites from worker bees can remove legs, which interferes with the mite’s ability to move and to hold on to bees, and also opens them up to dessication. It was recently shown just that 2-heptanone from worker mandibular glands, long thought to act as an alarm pheromone, actually is an anesthetic to invertebrates. A worker bite to a small wax moth larvae or the application of only 0.061 microliters of 2-heptanone to the back of Varroa mites causes temporary immobilization. This may be the main function of this chemical for the bees (Papachristoforou et al. 2014).
Not enough is known about different variables that influence the trait ‘proportion of chewed mites’ and repeated testing of colonies shows that it varies much more than we would like, which means it is influenced environmental effects. Perhaps when a large patch of adults emerge from the brood more mites fall passively. Another difficulty is that colonies that are effective in reducing mite populations often have insufficient mites on the sampling sheet, especially in the Spring. This is a good problem to have! But it necessitates choosing the Spring grafting sources depending on data from tests done the previous year. Better screening methods are needed that can be performed on colonies even if they have few mites. We are considering making initial selections based on the proportion of chewed mites and then using a secondary lab assay to observe grooming behavior of individuals or groups of workers to choose grafting source and drone colonies. We are also considering more regular testing for VSH.
Community stock evaluation:
We distributed 102 queens to 39 beekeepers in 2014. We received data from 23 beekeepers from IL, IN and OH that successfully introduced both of the queens into splits that came from one hive and evaluated them for a year, which represents 54 queens or 27 side-by-side comparisons of the two types of stock.
Beekeepers were given a single pair of queens to compare, except for one beekeeper that had four pairs of queens and another that had two pairs. The three commercial queen sources from Western states did not differ from one another in survival or mite levels so they were combined into one “commercial” stock for beekeeper evaluation. Likewise, the two IN queen sources did not differ from one another and were combined into one “IN” stock for evaluation. Most colony mortality occurred during the Winter; some of this was starvation and some was probably mite-related. A few colonies died before the Winter from unknown causes, perhaps queen failure. By March of 2015, only six of 27 commercial colonies (22%) were surviving. In contrast most of the IN colonies (15 of 27 or 55%) were still alive.

Figure 2. Evidence of Varroa-sensitive hygine (VSH). A drone comb infested with Varroa mites was inserted into one of our recent breeder colonies for 48 hours. This figure shows the before (top) and after (bottom).
Honey yields were estimated based on reports from 14 beekeepers over both years. Making the assumption that a medium depth super yields 30 lb. of honey and a shallow yields 20 lb., commercial source colonies produced an average of 11.7 lb. compared to 52.1 lb. for hives with IN queens, a 40.4 lb. difference. Most colonies did not produce surplus honey the first year, so most of the yield difference was caused by differences in survival. But there were also some differences in colony strength. Relative colony strength was reported in 12 cases; hives with IN queens were rated stronger for eight, weaker for two and equal strength for the other two.
Eight beekeepers reported on Varroa mite levels for both types of queens during 2014 or 2015 using either powdered sugar shakes, alcohol washes or sticky board sampling. One of these beekeepers reported a lower mite count in the commercial source hive (two thirds of the IN mite-biter level on a sticky board a month after introducing queens). Two beekeepers reported no mites in the IN colonies but found either six mites (powdered sugar shake) or 147 (sticky board) in the commercial colonies. The other seven beekeepers reported that the commercial source colonies averaged three-fold higher mite levels compared to those with mite-biter queens (2.7-fold higher for the eight comparisons).
Of the 11 beekeepers that stated a preference, 10 chose IN mite-biter queens over commercial-source queens in this blind comparison. One of those that preferred the mite-biters was comparing four pairs of queens. Two beekeepers mentioned that the colonies with IN queens were more defensive. One of these two said that the IN hive was slightly more aggressive than the other hives but that he preferred it because it was more productive. The other said that the commercial-source hive had very high mite counts (tested with alcohol wash) and was dead by Christmas. On the other hand, the IN hive had lower mite levels in the Fall, which were further reduced after Winter, but was “extremely defensive” and had 20 frames of bees by July of 2015.
This experiment was limited in scope because it only compared daughter queens from five mother queens. We also did not compare the “mite-biter” queens to other Midwestern stocks, which may have similar or better survival. We have seen other stocks that have relatively high proportions of chewed mites and believe this trait can be selected for in any genetically diverse population of bees. We do not know if this trait shows genetic dominance. One test of this would be to take virgin queens of low-biting stock and let them fly in our mating yard to see how their progeny do. There may also be environmental effects that influenced survival that had nothing to do with genotype or that interacted with genotype, such as exposure of queens to Nosema or virus, or the mating conditions. So we can’t make any strong conclusions but the large difference in winter survival and the beekeepers’ preference suggest that breeding for mite-grooming behavior in local stocks of bees will make beekeeping more sustainable in the North Central US. Stocks from this breeding program are being made available through the Heartland Honey Bee Breeders Cooperative. We think that it is important that queen breeders select for both VSH and grooming behavior in bees that have survived Northern Winters.
Acknowledgements
We would like to express our sincere gratitude to all of the beekeepers who took the time to evaluate queens, and to the Indiana Queen Breeders Association and Heartland Honey Bee Breeders Cooperative. The community stock evaluation was supported by a 2014 grant from the National Honey Board. The breeding program was supported in part by USDA grant 2008-35302-18803. This research was also partially funded by a USDA Sustainable Agriculture Research and Education (SARE) grant 3LNC08-295.
References.
Andino G.K., Hunt G.J. (2011) A scientific note on a new assay to measure honeybee mite-grooming behavior, Apidologie, 42, 481-484.
Arechavaleta-Velasco M., Guzmán-Novoa E. (2001) Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies, Apidologie 32, 157–174.
Arechavaleta-Velasco M.E., Alcala-Escamilla K., Robles-Rios C., Tsuruda J.M., Hunt G.J. (2012) Fine-scale linkage mapping reveals a small set of candidate genes influencing honey bee grooming behavior in response to Varroa mites, PLOS ONE 7(11), e47269. doi:10.1371/journal.pone.0047269.
Berry J.A., Owens W.B., Delaplane K.S. (2010) Small-cell comb foundation does not impede Varroa mite population growth in honey bee colonies. Apidologie 41:40–44.
Boecking O., Spivak M. (1999) Behavioral defenses of honey bees against Varroa jacobsoni Oud, Apidologie 30, 141-158.
Coffey M.F., Breen J., Brown M.J.F., McMullan J.B. (2010) Brood-cell size has no influence oh the population dynamics of Varroa destructor mites in the native western honey bee, Apis mellifera mellifera. Apidologie 41:522-530.
Ellis A.M., Hayes G.W., Ellis J.D. (2009) The efficacy of small cell foundation as a Varroa mite (Varroa destructor) control. Exp. Appl. Acarol. 47:311–316.
Guzmán-Novoa E., Emsen B., Unger P., Espinosa-Montaño L.G., Petukhova T. (2012) Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.), J. Invert. Path., 110, 314-320.
Harbo J.R., Harris J.W. (1999) Selecting honey bees for resistance to Varroa jacobsoni, Apidologie 30, 183–196.
Harbo J.R., Harris J.W. (2005) Suppressed mite reproduction explained by the behaviour of adult bees, J. Apic. Res. 44, 21–23.
Mondragon L, Spivak M, Vandame R (2005) A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 36: 345–358.
Moosbeckhofer R. (1992) Observations on the occurrence of damaged Varroa mites in natural mite fall of Apis mellifera carnica colonies, Apidologie 23, 523-531.
Papachristoforou A., Kagiava A., Papaefthimiou C., Termentzi A., Fokialakis N., Skaltsounis A.-L., Watkins M., Arnold G., Theophilidis G. (2014) The bite of the honeybee: 2-Heptanone secreted from honeybee mandibles during a bite acts as a local anaesthetic in insects and mammals, PLOS ONE, 7(10): e47432. doi:10.1371/journal.pone.0047432
Peng Y.S., Fang Y., Xu S., Ge L. (1987) The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite Varroa jacobsoni Oudemanns. J Invert Pathol 49: 54–60. doi: 10.1016/0022-2011(87)90125-x
Rinderer T.E., Harris J.E., Hunt G.J., de Guzman L.I. (2010) Breeding for resistance to Varroa destructor in North America, Apidologie 41, 409-424.
Rosenkranz P., Fries I., Boecking O., Sturner M. (1997) Damaged Varroa mites in the debris of honey bee (Apis mellifera L.) colonies with and without hatching brood, Apidologie 28, 427–437.
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103 Supplement: S96–S119 doi: 10.1016/j.jip.2009.07.016.
Seeley T.D., Downey D. (1998). Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J Econ Entomol 91:64-70.
Taylor M.A., Goodwin R.M., McBrydie H.M., Cox H.M. (2008) The effect of honeybee worker brood cell size on Varroa destructor infestation and reproduction. J. Apic. Res. 47:239–242.
Tsuruda J.M., Harris J.W., Bourgeois L., Danka R.G., Hunt G.J. (2012) High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees, PLOS ONE 7(11), e48276. doi:10.1371/journal.pone.0048276.
Zhou T., Yao J., Huang S.X., Huang Z.Y. (2001) Larger cell size reduces varroa mite reproduction. Proceedings of the American Bee Research Conference, American Bee Journal 141: 895-896.







