What Is It and Why Is It Important?
In June 2016, researchers from the University of Texas at Austin published the study “Gut Microbial Communities of Social Bees,” in Nature Reviews-Microbiology.
The microbial make-up of the human intestinal tract, called the microbiome, has received increasing attention over the past few years, particularly as it relates to the growing threat of antibiotic resistance. But bees? I talked with Department of Integrative Biology researcher and beekeeper, Nancy Moran, who co-authored the review, which included about seven years of studies describing microbiology of bee gut. Dr. Moran has taken a closer look – literally – at the features, shapes, and getting to name the genus-species – none had names – of the bacteria living inside of the intestinal tract of bees.
Why is it important to look into the microbiological makeup of the honey bee gut?
It is becoming apparent that the gut bacteria in animals are important to health, based on studies in mice, humans, insects and other groups. Therefore it is very likely they are also important in bees. In addition, our research has shown us that honey bees have a very consistent set of bacterial species, present in all honey bee workers worldwide. The microorganisms in the guts of animals can ben-efit their hosts by helping to digest food, detoxifying harmful molecules, providing essential nutrients, pro-tecting against invading pathogens and parasites, and modulating development and immunity. However, although the importance of the gut microbiota is becoming increasingly appreciated, the processes that govern these microbial communities are largely unclear.
 Since 2006, honey bee colonies have been suffering from a high mortality. Once we learn more about the internal bacteria and their role – helpful or harmful – we can look at external environmental factors like food supply, treatments and toxins on the effect on the bacteria and, therefore, the effect on the bee. All of these stressors might be modulated by their gut microorganisms. So studying this area can help us understand honey bee biology broadly, and contribute to a better understanding of the role of bacteria, specifically, in the digestive tract.
Since 2006, honey bee colonies have been suffering from a high mortality. Once we learn more about the internal bacteria and their role – helpful or harmful – we can look at external environmental factors like food supply, treatments and toxins on the effect on the bacteria and, therefore, the effect on the bee. All of these stressors might be modulated by their gut microorganisms. So studying this area can help us understand honey bee biology broadly, and contribute to a better understanding of the role of bacteria, specifically, in the digestive tract.
The idea is that the bee gut is a simple model, with similar processes, can help us better understand how gut communities work in general. This can, of course, not only help us become better beekeepers, but it can also help us gain insights into how a much more complicated and variable system like the human digestive tract works. The National Institutes of Health, who funded part of the research, is very interested in this area.
What were your key findings? Were they what you expected?
A central finding, and one that motivated the further studies, was that honey bee workers have a very consistent set of gut bacteria that make up most of the microbes in the gut – often 99% or more. I first became aware of this when I participated in a collaborative study in 2007 that tried to use new sequencing methods to identify possible pathogens that might be associated with Colony Collapse Disorder. It turned out that no clear pathogen was identified. However, one thing that stood out was that all of the samples had the same set of bacteria. Subsequent sampling showed that these bacteria were in all Apis mellifera workers worldwide, and some of these species are also present in other species, such as the Asiatic honey bee Apis cerana, and in bumble bee species.
Based on this earlier research, we have been trying to understand the role that the makeup of gut bacteria plays in the honey bee, whether different strains improve bee health or hurt it. Although there are only about eight species of bacteria that we consistently find in the digestive tract of the honey bee, each species has many strains with lots of genetic variation and different pathways. We used several kinds of experiments.
We took pupae out of the frame when they were close to emerging and put them in a clean dish. We found that they don’t acquire microbiota in the pupal stage. We established what kinds of environmental exposure establishes their microbiota. We took new bees with a “clean” gut, marked them, and put into hive and sampled them over time. For a few days, the microbiota is erratic, but around days three to four, a consistent pattern emerges of what we call the core microbiota of the adult honey bee gut.
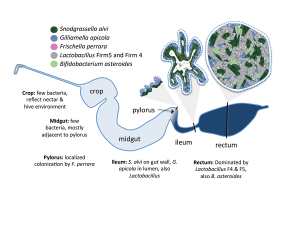
We were also able to determine where the microbes resided in the digestive tract. Few bacteria were found in the front and midgut. This started to change near the pylorus, which connects the midgut to the hindgut. The ileum is where we found the microbial population really start to colonize. The rectum showed the highest concentration of microbial population. It was important to determine this because then we can look at the role of those bacteria in the specific location. Is it to fight other disease-causing bacteria? Is it to aid in fermentation of food and digestion? Because the honey bee gut is host to such a small number of microbes, we can identify their roles more easily.
Scientific studies have shown that there is clear evidence that the microbiome can interact with at least some pathogens, such as trypanosomatid parasites like Crithidia bombi. This seems to be true in both honey bees and bumble bees. The genomes of the bacteria tell us that they are able to break down plant macromolecules that are present in the cell walls of the pollen grains. So, it’s possible that the bees can extract additional energy that affects the economy of the hive (in addition to using sugars from nectar). They can also utilize a range of sugars, not just sucrose, fructose and glucose, but also some such as mannose and arabinose and xylose that are present in plant cells walls and that have been shown to be toxic to bees.
How do the bees acquire and share gut bacteria?
Sociality, which includes the sharing of the hive environment by a social group, is central to the between-host transmission of the bee gut microbiota. After pupating in capped cells inside the colony, adult honey bees emerge that have germ-free guts. If removed manually from the honeycomb cells and maintained under sterile laboratory conditions, the guts of the honey bees do not acquire substantial microbial communities. Newly-emerged bees that chew out of the honeycomb cells on their own may be inoculated by residual gut symbionts on the frame surface.
Initially, the microbial community is small and erratic in composition, dominated by environmental bacteria and lacking differentiation between regions of the gut. We found that by the third day, though, the microbial communities contain more than 107 bacteria mostly from the characteristic species of the bee gut, and the ileum and rectum begin showing “normal” community compositions. These microbial communities plateau at approximately 109 bacteria by the eighth day. Once established, gut microbial communities are generally stable as workers transition through different behavioral stages. This shift, incidentally, from an initial erratic microbial community to one dominated by “adult” bacteria, largely mirrors the development of the gut microbiota in human infants.
The establishment of a stable microbial community occurs before worker bees leave the hive, which implies that transmission occurs through other bees in the colony or hive components, such as wax surfaces. In experimental tests, the typical gut microbial communities developed with the highest likelihood when bees were directly exposed to older bees or were fed their macerated hindguts. Based on experiments that manipulated potential transmission sources, oral trophallaxis, a common behavior for communication and food transfer, is not a major route for transmission, which is consistent with observations that the foregut harbors few bacteria. A fecal route seems important, particularly for Snodgrassella alvi, Gilliamella apicola and Frischella perrara, although members of the gut microbial community may also be acquired through contact with hive components that have been in recent contact with live bees. Species in the Acetobacteraceae family might be transmitted through the stored pollen food supply.
Were there differences in the microbiome of worker bees vs. drones vs. queens?
Yes. We focused on workers, but other studies have shown that queens have very different microbiomes. Drones are more similar to workers, though not exactly the same. The workers have a microbiome that reflects an emphasis on fermentation of the food products – nectar and pollen – so it makes sense that queens and drones might not have this same gut community.
Adult workers have a relatively stable set of bacterial species in their gut compared with drones or queens, and when compared with other insects generally. Nonetheless, even workers of the same age in a col-ony can harbor very different proportions of the core species of bacteria in the gut. Colonies might also undergo age-related or seasonal shifts in the relative pro-portions of the core species of bacteria. The extent to which these shifts are specific to particular geographic regions or environmental conditions is unclear, and is something we are interested in further researching.
Do you think this research can shed light into factors that might contribute to the health of honey bee colonies and possibly even the decline of bee populations? How so?
We think that the microbiome is central to maintaining health and that a disrupted microbiome makes the host susceptible to a variety of problems. Normally bees can acquire a normal, healthy, microbiome. However, we are starting to see that some practices could interfere with this. The most obvious is antibiotic application.
We found that the gut microbiome of U.S. honey bees has a very high incidence of tetracycline resistance, reflecting the use of tetracycline in U.S. beekeeping for the past 50-plus years for the treatment and prevention of foulbrood. These resistance genes were not found in guts of honey bees from countries that don’t use antibiotics in beekeeping. Potentially, the use of antibiotics or other treatments could interfere with the normal microbiota and make colonies susceptible to various pathogens or other stressors—but I emphasize the word potentially. So far, we don’t have results that firmly point to ways that beekeeping practices should be changed. We are more focused on the basic biology of these organisms. But we are hoping that the work will provide a better general understanding of the factors that govern bee health and may eventually lead to healthier bees because of better-informed beekeeping. One general idea we had was to look at differences in the bee microbiome based on geography. We could discover, for example, that certain environmental situations are better for bees living in Texas or New England, and beekeepers could benefit from those findings in terms of optimizing their surroundings.
Looking ahead, there may be some direct implications for the way commercial and backyard beekeepers care for their bees. Without getting ahead of ourselves, we can see that improper use of antibiotics for sure can have a major impact on beekeeping, just as it has had in humans. Antibiotic stewardship efforts are underway around the world – the right drug, for the right bug, at the right time – for humans, livestock, and yes, even bees.
There has been a lot of news over the past several years about the diversity of bacterial life in the human digestive tract. What are some of the similarities and differences between human and bee gut microbial communities? Can your findings be translated to humans, or help inform future research into human gut microbiology?
Indeed, one of our main motivations, besides understanding more about bee health, is that the bee gut microbiota is a good model for many aspects of the human gut microbiota. In both, there are specialized gut bacteria that are transmitted directly from individual to individual during social interactions or group living.
In honey bees, workers obtain their gut bacteria after they emerge from the pupal stage to become adults in the hive. Research has shown that the gut bacteria community is erratic for the first few days, with many environmental bacteria temporarily present. But this stabilizes by day five or so. This process is parallel to that seen in newborn babies, which initially have an erratic gut community that later stabilizes to become dominated by species specialized to live in the gut. In both bees and humans, the bulk of the bacteria are present in the hindgut, past the location of most digestion and absorption, so the communities are living in a low-nutrient environment and extracting energy from the more recalcitrant compounds such as plant polymers like lignocellulose or pectin. And in both cases, the communities have a lot of strain level diversity, with very closely related bacteria that differ in their genomes in ways that might affect the hosts.
There are also some parallels in how the microbes interact with the innate immune system, as both bees and humans share some of the same immune mechanisms that are found in all animals. For example, all animals and plants make small peptides – antimicrobial peptides – whereby normal gut bacteria develop resistance. Antimicrobial peptides help maintain gut symbiosis. We can think of this as a “truce” between host and normal bacteria. Snodgrassella is present on the gut wall the host and prevent other, more harmful, bacteria from invading. However, we don’t want the bacteria to cross over that gut wall and into the host; Snodgrassella can live and not penetrate into the host and cause infections and could cause a protective layer against a more harmful infection, a pathogen that you want to keep out.
What are areas of future research in terms of the honeybee gut microbiota?
In addition to looking inside the gut of honey bees alive today, University of Texas researchers are looking at the evolution of bacteria living inside of the digestive system going back generations as bees have evolved. They have collected Apis species from around the world in an effort to identify the common ancestor 80 million years ago, and the original bacteria living in the gut that has evolved right alongside with the bee as the host. We are also looking at researching the genetics of bacteria and the effect of specific genes. Most bacteria cannot colonize in the honeybee gut, and the ones that have are very specialized. We wonder why that is the case and would like to learn more.
Rebecca Novak Tibbitt is a health communications consultant with RNT Communications, LLC.