By: Clarence Collison
Little is known about how waggle dance followers are able to read the waggle dance in the darkness of the hive.
Honey bees communicate to nestmates locations of resources, including food, water, tree resin (propolis) and nest sites, by making waggle dances. Dances are composed of repeated waggle runs, which encode the distance and direction vector from the hive or swarm to the resource. Distance is encoded in the duration of the waggle run, and direction is encoded in the angle of the dancer’s body relative to vertical (Schürch et al. 2013).
The waggle dance can be regarded as a repetition of movements consisting of a waggle “run” and a return run. During the waggle run, the dancer swings her body from side to side in a pendulum-like manner, 13-15 times per second, and she produces dance sounds by vibrating her wings dorsoventrally (Wenner 1962). The bee moves her body continuously forward, but her legs do not move at all or perform only a few slow-motion strides (Tautz et al. 1996). In the return run, the dancer circles back to start a new sequence. Some parameters of the waggle run are correlated with the location of the advertized feeding site. The angle between the sun’s azimuth and the direction to food in the field equals the angle between gravity and waggle run, which is called waggle run angle. The distance to the food source is indicated in the duration of the waggle run: the longer the duration of the waggle run, the further away is the feeding site (von Frisch 1967). Dance followers mostly accompany the dancing bee. The interaction between dancers and dance followers can be broken up into the following successive steps: First, bees motivated to follow a dancer detect, localize and approach the dancer (Tautz and Rohrseitz 1998). Second, they accompany her, often for many circuits (Esch and Bastian 1970), and thus become dance followers. Third, after following a number of dances, they often fly out and find the indicated food source.
Little is known about how waggle dance followers are able to read the waggle dance in the darkness of the hive. Initial observations showed that not all of the bees that appear to be dance followers behave the same. Some bees maneuver themselves behind the dancer, while others do not. The paths of a single dancer, trained to an artificial food source, and her followers were traced during the waggle runs. The success of these dance followers was compared to their position relative to the dancer. The results of this study show that during a waggle run a dance follower must position itself within a 30º arc behind the dancer in order to obtain the dance information. The results suggest that bees are using the position of their own bodies to determine direction (Judd 1994).
The behavior of bees surrounding a dancing bee was studied, using two colonies in observation hives in a shaded part of an apiary. Video recordings and macrophotography were used to view an area of the dance field. Two distinct behaviors were recognized: that of followers and that of attendants. The attendants stood around the dance field with their antennae stretched towards the dancer, and only occasionally moved with the dancer. Followers continuously ran with the dancer, keeping their heads within the border of the dancer’s figure-eight paths at all times. The angle between the body of the follower and that of the dancer was 90º during most of the dance, except at the exit of the waggle run. At that time the follower had to cross over to the opposite side of the dancer. The distance between the head of the follower and the dancer’s body was nearly always smaller (1,758 cases out of a total of 1,882) than the length of an extended antenna. During the return run the follower touched the dancer with antennae most of the time, whereas during the waggle run the followers intermittently touched the dancer. Either one bee (81 % of cases) or two bees (18%) followed the dance simultaneously. The second follower and all other bees were usually pushed out of the follower’s position because of a lack of space at the inner side of the dancer (Božič and Valenlinčič 1991).
Biesmeijer and Seeley (2005) studied the extent to which worker honey bees acquire information from waggle dances throughout their careers as foragers. Small groups of foragers were monitored from time of orientation flights to time of death and all in-hive behaviors relating to foraging were recorded. In the context of a novice forager finding her first food source, 60% of the bees relied, at least in part, on acquiring information from waggle dances (being recruited) rather than searching independently (scouting). In the context of an experienced forager whose foraging has been interrupted, 37% of the time the bees resumed foraging by following waggle dances (being reactivated) rather than examining the food source on their own (inspecting). And in the context of an experienced forager engaged in foraging, 17% of the time the bees initiated a foraging trip by following a waggle dance. Such dance following was observed much more often after an unsuccessful than after a successful foraging trip. Successful foragers often followed dances just briefly; perhaps to confirm that the kind of flowers they had been visiting were still yielding forage. Overall, waggle dance following for food discovery accounted for 12-25% of all interactions with dancers (9% by novice foragers and 3-16% by experienced foragers) whereas dance following for reactivation and confirmation accounted for the other 75-88% (26% for reactivation and 49-62% for confirmation). They concluded that foragers make extensive use of the waggle dance not only to start work at new, unfamiliar food sources but also to resume work at old, familiar food sources.
“Hydrocarbons emitted by waggle-dancing honey bees are known to reactivate experienced foragers to visit known food sources.”
The behavior of 38 honey bee dance followers and the patterns of antennal contact between followers and dancer were monitored during ten waggle runs for a feeding site 1200 meters from the hive. The analysis was restricted to waggle runs with a maximum of five followers, allowing the followers to choose between different positions around the dancer. At the beginning of the waggle run, followers are rather evenly spaced around the dancer. During the waggle run, the followers tend to accumulate at the rear end of the dancer. At the end of the waggle run, all followers are found in a ±60º arc behind the dancer. The body orientation angles of the followers depend on their position relative to the dancer. The follower bees have intense antennal contact with the dancer. At least one temporal parameter of the contact pattern may inform the followers about their position relative to the dancer, may guide the dance followers to the rear end of the dancer and may allow them to extract information about the location of the food source advertised by the dance. The role of antennal contact for dance communication appears to have been underestimated in previous studies (Rohrseitz and Tautz 1999).
Tautz (1996) reported that the nature of the floor on which the bees dance has considerable influence on the recruitment of nestmates to a food source. Dancers on combs with open empty cells recruit three times as many nestmates to a food source as dancers on capped brood cells.
Honey bee foragers can follow waggle dances (social information) to obtain vector information about the location of profitable food sources or they can use route memories (private information) acquired during previous foraging trips. The relative use of social information versus private information is poorly understood. It is hypothesized that social information should be prioritized when the use of private information has a low benefit. Grüter and Ratnieks (2011) tested this hypothesis by training foragers to a high-quality 2 M sucrose feeder, which subsequently became unrewarding. As foragers continued to experience zero reward from their private route information they increased the time spent following waggle dances advertising an alternative food source with the same odor. A significant proportion of foragers successfully switched to the food source indicated by dances. Overall, trained foragers showed a strong attachment to the known but currently unrewarding feeder, even after repeatedly following dances advertising a profitable alternative. Successful recruits to the novel food source advertised by the waggle dances had more social information about this source in that they had followed dances for longer. Their results suggest that honey bee foragers follow a strategy that is conservative in terms of switching from one food patch to another.
Hydrocarbons emitted by waggle-dancing honey bees are known to reactivate experienced foragers to visit known food sources. Gilley (2014) investigated whether these hydrocarbons also increase waggle-dance recruitment by observing recruitment and dancing behavior when the dance compounds are introduced into the hive. If the hydrocarbons emitted by waggle-dancing bees affect the recruitment of foragers to a food source, then the number of recruits arriving at a food source should be greater after introduction of dance compounds versus a pure-solvent control. This prediction was supported by the results of experiments in which recruits were captured at a feeder following introduction of dance compounds into a hive. This study also tested two nonexclusive behavioral mechanism(s) by which the compounds might stimulate recruitment; 1) increased recruitment could occur by means of increasing the recruitment effectiveness of each dance and/or 2) increased recruitment could occur by increasing the intensity of waggle-dancing. These hypotheses were tested by examining video records of the dancing and recruitment behavior of individually marked bees following dance compound introduction. Comparisons of numbers of dance followers and numbers of recruits per dance and waggle run showed no significant differences between dance compound and solvent-control introduction, thus providing no support for the first hypothesis. Comparison of the number of waggle-dance bouts and the number of waggle runs revealed significantly more dancing during morning dance-compound introduction than morning solvent-control introduction, supporting the second hypothesis. These results suggest that the waggle-dance hydrocarbons play an important role in honey bee foraging recruitment by stimulating foragers to perform waggle dances following periods of inactivity.
Thom et al. (2007) used solid-phase microextraction and gas chromatography coupled with mass spectrometry to show that waggle-dancing bees produce and release two alkanes, tricosane and pentacosane, and two alkenes, Z-(9)-tricosene and Z-(9)-pentacosene, onto their abdomens and into the air. Non-dancing foragers returning from the same food source produce these substances in only minute quantities. Injection of the scent significantly affects worker behavior by increasing the number of bees that exit the hive. The results of this study suggest that these compounds are semiochemicals involved in worker recruitment. By showing that honey bee waggle dancers produce and release behaviorally active chemicals, this study reveals a new dimension in the organization of honey bee foraging.
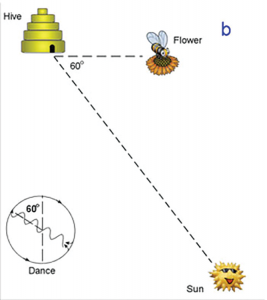
Direction of the food source is indicated by the direction the dancer faces during the straight portion of the dance when the bee is waggling. If she waggles while facing straight upward, than the food source may be found in the direction of the sun.
From the honey bee dances, human observers can read the distance and direction of the food source. When foragers collect food in a short, narrow tunnel, they dance as if the food source were much farther away. Dancers gauge distance by retinal image flow on the way to their destination. Their visually driven odometer misreads distance because the close tunnel walls increase optic flow. Esch et al. (2001) examined how hive mates interpret these dances. They were able to show that recruited bees search outside in the direction of the tunnel at exaggerated distances and not inside the tunnel where the foragers come from. Thus, dances must convey information about the direction of the food source and the total amount of image motion en route to the food source, but they do not convey information about absolute distances. They also found that perceived distances on various outdoor routes from the same hive could be considerably different. Navigational errors are avoided as recruits and dancers tend to fly in the same direction. Reported racial differences in honey bee dances (von Frisch 1967) could have arisen merely from differences in the environments in which these bees flew.
The sound and air flows generated during the waggle dance by the dancer’s wing and abdominal vibrations have been implicated as important cues for the bees following the dancer. To understand the neural mechanisms of honey bee dance communication, Tsujiuchi et al. 2007, analyzed the anatomy of the antenna and Johnston’s organ (JO) in the pedicel of the antenna, as well as the mechanical and neural response characteristics of antenna and JO to acoustic stimuli, respectively. The honey bee JO consists of about 300-320 scolopidia (fundamental unit of a mechanoreceptor organ in insects, sensitive to sound (vibrations of the air) or substrate vibrations) connected with about 48 cuticular “knobs” around the circumference of the pedicel. Each scolopidium contains bipolar sensory neurons with both type I and II cilia. These neurons convert mechanical vibrations into a nerve impulse. The mechanical sensitivities of the antennal flagellum (antennal segments beyond the second segment) are specifically high in response to low but not high intensity stimuli of 265-350 Hz frequencies (Hz = hertz, one vibration cycle/second). The honey bee flagellum is a sensitive movement detector responding to 20 nm tip displacement. Furthermore, the JO neurons have the ability to preserve both frequency and temporal information of acoustic stimuli including the “waggle dance” sound. Intriguingly, the response of JO neurons was found to be age-dependent, demonstrating that the dance communication is only possible between aged foragers. These results suggest that the matured honey bee antennae and JO neurons are best tuned to detect 250-300 Hz sound generated during the “waggle dance” from the distance found in a dark hive, and that sufficient responses of the JO neurons are obtained by reducing the mechanical sensitivity of the flagellum in a near-field of dancer.
Waggle-dancing honey bees produce vibratory movements that may facilitate communication by indicating the location of the waggle dancer. However, an important component of these vibrations has never been previously detected in the comb. Nieh and Tautz (2000) developed a method of fine-scale behavioral analysis that allowed them to analyze separately comb vibrations near a honey bee waggle dancer during the waggle and return phases of her dance. They simultaneously recorded honey bee waggle dances using digital video and laser-Doppler vibrometry and performed a behavior-locked Fast Fourier Transform analysis on the substratum vibrations. This analysis revealed significantly higher-amplitude 200-300 Hz vibrations during the waggle phase than during the return phase. They found no significant differences in the flanking frequency regions between 100-200 Hz and 300-400 Hz. They recorded peak waggle phase vibrations from 206 to 292 Hz with a mean of 244 Hz. Therefore, the dance followers likely obtain cues from both sound and substrate vibrations.
References
Biesmeijer, J.C. and T.D, Seeley 2005. The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 59: 133-142.
Božič, J. and T. Valentinčič 1991. Attendants and followers of honey bee waggle dances. J. Apic. Res. 30: 125-131.
Esch, H. and J.A. Bastian 1970. How do newly recruited honeybees approach a food site? Z. Vergl. Physiol. 68: 175-181.
Esch, H.E., S. Zhang, M.V. Srinivasan and J. Tautz 2001. Honeybee dances communicate distances measured by optic flow. Nature 411: 581-583.
Gilley, D.C. 2014. Hydrocarbons emitted by waggle-dancing honey bees increase forager recruitment by stimulating dancing. PLoS ONE 9(8):e105671. doi:10.1371/journal.pone.0105671.
Grüter, C. and F.L.W. Ratnieks 2011. Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 81: 949-954.
Judd, T.M. 1994. The waggle dance of the honey bee: Which bees following a dancer successfully acquire the information? J. Insect Behav. 8: 343-354.
Nieh, J.C. and J. Tautz 2000. Behaviour-locked signal analysis reveals weak 200-300 Hz comb vibrations during the honeybee waggle dance. J. Exp. Biol. 203: 1573-1579.
Rohrseitz, K. and J. Tautz 1999. Honey bee dance communication: waggle run direction coded in antennal contacts? J. Comp. Physiol. A 184: 463-470.
Schürch, R., M.J. Couvillon, D.D.R. Burns, K. Tasman, D. Waxman and F.L.W. Ratnieks 2013. Incorporating variability in honey bee waggle dance decoding improves the mapping of communicated resource locations. J. Comp. Physiol. A 199: 1143-1152.
Tautz, J. 1996. Honeybee waggle dance: recruitment success depends on the dance floor. J. Exp. Biol. 199: 1375-1381.
Tautz, J. and K. Rohrseitz 1998. What attracts honeybees to a waggle dancer? J. Comp. Physiol. A 183: 661-667.
Tautz, J., K. Rohrseitz and D.C. Sandeman 1996. One-strided waggle dancer in bees. Nature 382: 32.
Thom, C., D.C. Gilley, J. Hooper and H.E. Esch 2007. The scent of the waggle dance. PLoS Biol. 5(9):e228. doi:10.1371/journal.pbio.0050228.
Tsujiuchi, S., E. Sivan-Loukianova, D.F. Eberl, Y. Kitagawa and T. Kadowaki 2007. Dynamic range compression in the honey bee auditory system toward waggle dance sounds. PLoS ONE 2(2):e234.
doi:org/10.1371/journal.pome.0000234.
von Frisch, K. 1967. The Dance Language And Orientation Of Bees, Harvard University Press, Cambridge, MA., 566 pp.
Wenner, A.M. 1962. Sound production during the waggle dance of the honey bee. Anim. Behav. 10: 79-95.
Clarence Collison is an Emeritus Professor of Entomology and Department Head Emeritus of Entomology and Plant Pathology at Mississippi State University, Mississippi State, MS.








