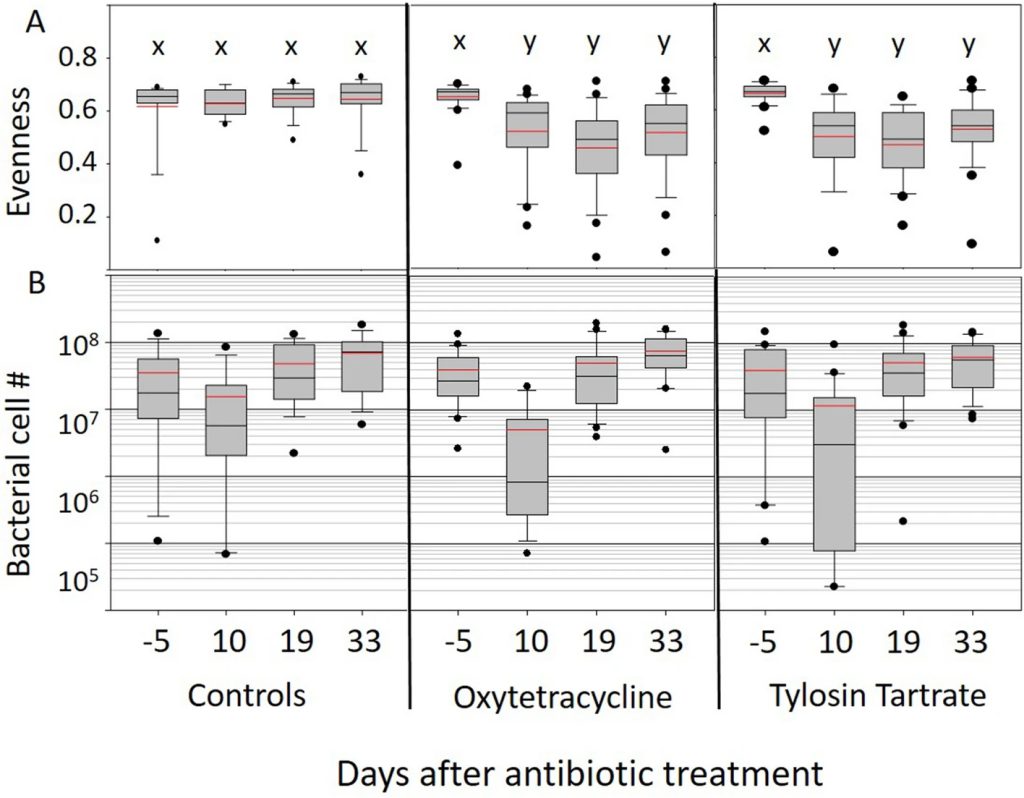
Two metrics that illustrate changes in the gut microbiome over time associated with antibiotic treatment. The top panels display Shannon Evenness, a metric of gut microbial diversity with values ranging from complete species dominance (0.0) to absolute evenness (1.0). Lower panels show microbiome size, determined by BactQuant, and expressed as total bacterial cell number. The x-axis lists four time periods sampling control colonies and colonies treated with either oxytetracycline or tylosin tartrate; five days before treatment, ten days after treatment, nineteen days after treatment, and thirty-three days after treatment. Grey boxes contain 50% of the variation, red line is the mean and black the median, whiskers are at 10 and 90% and dots are outliers. Within each panel, box plots with the same letter do not differ (p < 0.05).
A longitudinal field study of commercial honey bees shows that non-native probiotics do not rescue antibiotic treatment and are generally not beneficial.
We are here to share current happenings in the bee industry. Bee Culture gathers and shares articles published by outside sources. For more information about this specific article, please visit the original publish source: A longitudinal field study of commercial honey bees shows that non-native probiotics do not rescue antibiotic treatment, and are generally not beneficial | Scientific Reports (nature.com)
- Kirk E. Anderson,
- Nathan O. Allen,
- Duan C. Copeland,
- Oliver L. Kortenkamp,
- Robert Erickson,
- Brendon M. Mott &
- Randy Oliver
Scientific Reports volume 14, Article number: 1954 (2024)
Abstract
Probiotics are widely used in agriculture including commercial beekeeping, but there is little evidence supporting their effectiveness. Antibiotic treatments can greatly distort the gut microbiome, reducing its protective abilities and facilitating the growth of antibiotic resistant pathogens. Commercial beekeepers regularly apply antibiotics to combat bacterial infections, often followed by an application of non-native probiotics advertised to ease the impact of antibiotic-induced gut dysbiosis. We tested whether probiotics affect the gut microbiome or disease prevalence, or rescue the negative effects of antibiotic induced gut dysbiosis. We found no difference in the gut microbiome or disease markers by probiotic application or antibiotic recovery associated with probiotic treatment. A colony-level application of the antibiotics oxytetracycline and tylosin produced an immediate decrease in gut microbiome size, and over the longer-term, very different and persistent dysbiotic effects on the composition and membership of the hindgut microbiome. Our results demonstrate the lack of probiotic effect or antibiotic rescue, detail the duration and character of dysbiotic states resulting from different antibiotics, and highlight the importance of the gut microbiome for honeybee health.








