By: Vaughn Bryant
I have been examining pollen in honey samples for over 50 years and I am just now getting good at it! In spite of that, I still get calls and emails from beekeepers who ask if they can come visit my lab on a weekend and learn how to examine the pollen in their honey. The answer of course is that it actually takes a great deal of knowledge about plants, pollen, honey bees, and ecology. Honey bees can use more than 350,000 plant species worldwide to collect either pollen or nectar. Each of those plants produces a unique type of pollen, which makes honey analysis using pollen difficult. There are also other problems in trying to identify honey types using pollen.
When examining honey samples scientists who use pollen (palynologists) to identify the type of honey encounter many problems. First, we have learned that field observation of blooming plants by beekeepers, who identify those plants as being the source of their honey, is most often incorrect. After examining several thousand honey samples from hives all over the world we have discovered that more than 70% of those field identifications of honey types are incorrect. Second, experimental data reveal that not all honey bees are created equal. Some (56%) are better at collecting nectar and removing vast amounts of pollen during their return flight to the hive. The other 44% of bees in a hive are efficient at collecting nectar but are inefficient at removing the pollen from the nectar. We have also learned that the size and shape of a pollen grain often determines how efficiently honey bees can remove that pollen type from the nectar sources they collect; the larger the pollen grain the more efficient bees are at removing it from nectar. Third, a growing number of beekeepers and honey producers partially or completely filter their comb honey before selling it. Fourth, we have examined various processing techniques currently used to extract debris from honey and found that most techniques also remove various amounts of pollen. Finally, even when honey samples are correctly processed and their pollen contents are carefully noted, the resulting pollen data may not provide accuracy as to the primary nectar sources used to produce the honey. This last point depends on the skill of the scientist examining the honey and the use of pollen coefficient tables.
During the early 1940s, two scientists working for the USDA in California, Frank Todd and George Vansell, examined the relationship between the pollen in floral nectar sources and how that related to pollen in honey. Their research began when they discovered that bee colonies survived, but would not reproduce when fed only sugar syrup. Once pollen was added to their diet, the queen bee began egg laying within 12 hours. Their research began by collecting and examining over 2,600 individual samples of nectar. They had three major goals. Their first goal was to determine the number of pollen grains found in one cubic centimeter of nectar from various plant species. The second goal was to determine if the number of pollen grains naturally occurring in nectar samples matched the number of pollen grains found in the honey stomachs of bees that foraged on those flowers. The third goal was to discover how efficiently honey bees could remove pollen from the nectars they collected. Their published data showed that not all plants contribute pollen equally to nectar and honey. They effectively demonstrated that there is not a 1:1 relationship between a honey bee’s collection of a plant’s nectar and the percentages of pollen types found in honey. Their research became the foundation for the later development of melissopalynology (study of pollen in honey), and pollen coefficient values, which are now used to correct for these many variables.
 Pollen can become part of honey in a number of ways. When a honey bee lands on a flower, some of the flower’s pollen falls into the nectar that is sucked up by the bee and stored in her stomach. At the same time, other pollen grains can become attached to the “hairs”, legs, antenna, and even the eyes of visiting bees. Later, some of the pollen that was sucked into her stomach will be regurgitated with the collected nectar and deposited into open comb cells of the hive. While still in the hive, that same honey bee may groom her body in an effort to remove the entangled pollen. During that process pollen can fall directly into open comb cells or onto areas of the hive where other bees may track it into regions of the hive where unripe honey is still exposed. Airborne pollen is another potential source of pollen in honey. Airborne pollen produced by wind-pollinated plants, not usually visited by honey bees for nectar, can enter a hive on wind currents. These airborne pollen grains are usually few in number, when compared to the pollen carried into the hive by worker bees; nevertheless, those pollen types regularly enter a hive on air currents and can settle in areas where open comb cells are being filled with nectar.
Pollen can become part of honey in a number of ways. When a honey bee lands on a flower, some of the flower’s pollen falls into the nectar that is sucked up by the bee and stored in her stomach. At the same time, other pollen grains can become attached to the “hairs”, legs, antenna, and even the eyes of visiting bees. Later, some of the pollen that was sucked into her stomach will be regurgitated with the collected nectar and deposited into open comb cells of the hive. While still in the hive, that same honey bee may groom her body in an effort to remove the entangled pollen. During that process pollen can fall directly into open comb cells or onto areas of the hive where other bees may track it into regions of the hive where unripe honey is still exposed. Airborne pollen is another potential source of pollen in honey. Airborne pollen produced by wind-pollinated plants, not usually visited by honey bees for nectar, can enter a hive on wind currents. These airborne pollen grains are usually few in number, when compared to the pollen carried into the hive by worker bees; nevertheless, those pollen types regularly enter a hive on air currents and can settle in areas where open comb cells are being filled with nectar.
Pollen is an essential tool in the analyses of honey. The type of pollen indicates the floral sources utilized by bees to produce honey. As a result, pollen frequency is sometimes used to label a honey sample as to the major and minor nectar sources. This information has important commercial value because some consumers prefer honey made from certain plants, which command a premium price (i.e., white acacia, sourwood, sage, tupelo, buckwheat, or citrus honey). Even non-premium grades of honey often need to be examined for legal reasons because they must be correctly labeled as to type before being marketed. Only by identifying and quantifying, the pollen in honey can the full range of plant taxa be known and the honey’s actual floral sources be correctly labeled. Another reason that pollen analyses of honey are often required is to determine the honey’s geographical origin. The combination of airborne and insect-pollinated taxa found in a honey sample will often produce a pollen spectrum that is unique for a specific geographical region. Because of trade agreements, import tariffs, and legal trade restrictions, most of the leading honey-producing nations of the world require accurate labeling of honey before it can be sold. The United States and Canada are exceptions since neither requires truth in labeling for honey samples. Other reasons to establish the origin of honey is because some prefer to buy “local” honey. The pollen contents can confirm or reject honey labeled as being local!
The honey bee’s filtering process is rapid and effective. The bee sucks nectar into a slender tube that ends in the bee’s abdomen where it becomes an enlarged thin-walled sac called the honey stomach. This honey stomach is greatly distensible and can expand to hold large amounts of nectar. Once in the honey stomach, the nectar flows over the proventriculus that filters and controls the entrance of food into the bee’s stomach. The front end of the proventriculus, called the honey stopper, projects into the bee’s honey stomach like the neck of a bottle. At its back end is an x-shaped opening consisting of four, thick, triangular-shaped, muscle-controlled lips. Nectar in the honey stomach is passed back and forth into the funnel-shaped proventriculus. This process filters the nectar and removes debris such as pollen grains and the fungal spores that cause foul brood. From time to time people get alarmed about a phenomenon referred to as “yellow rain.” When large numbers of bees forage on nectars that are full of pollen, the rapid removal of those pollen grains from their honey stomachs and the resulting defecation by those swarms of bees can appear as “yellow rain” spots on leaves, cars, sidewalks, or buildings.
Experiments with caged honey bees, fed only a mixture of pollen and sugar water, revealed just how efficient the proventriculus is at removing pollen. Newly produced honey by these caged bees revealed two things. First, the bees were able to remove vast amounts of pollen before depositing the contents of their honey stomachs into empty comb cells. Second, it revealed that larger pollen grains are removed much more efficiently than smaller pollen grains. Thus, large pollen grains such as those from fireweed, magnolia, tulip tree, and tallow tree are removed much quicker than smaller pollen grains like those of many clovers, willows, chestnut, forget-me-nots, eucalyptus, and mulberry.
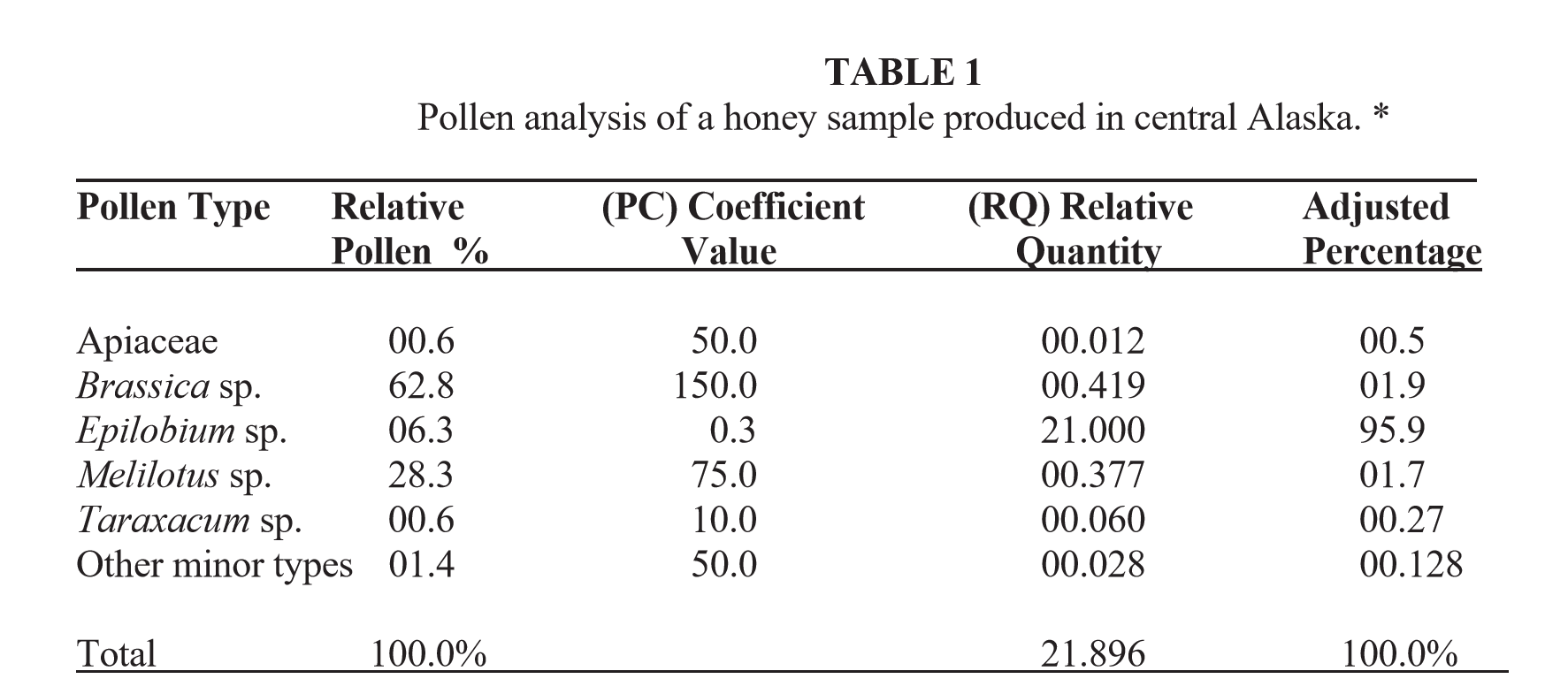
Rex Sawyer, was one of the foremost early melissopalynologist (analysts who look at pollen in honey) in the United Kingdom and Europe. He began his lifelong interest in honey pollen studies during the 1930s and published several books on pollen and honey. Included in his last book is a table listing the numerical pollen coefficient (PC) values that he developed for a number of nectar sources found mostly in the U.K. and Europe. PC values are expressed as the expected number of pollen grains per gram of honey. By following Sawyer’s formula, and applying his and other PC values to the relative pollen percentages found in any honey sample, one can determine the “actual” floral identities and “true nectar sources” of many honey types. He also said that he believes PC values can be applied to samples from almost any region of the world in order to “correct” the relative pollen percentages in honey. The basis for our current use of PC values in North America come from the data he generated as well as data from many other published sources. The only problem with PC values is that bees can collect nectar or pollen from more than 350,000 potential plant species worldwide. Unfortunately, we do not have PC values for most of those plants. Fortunately, for many of the primary honey types we do have PC values. For exotic honey types, we can often make reasonable guesses as to the PC values based on the size of the pollen grains that remain in the honey. Granted, in most of those exotic types we are “guessing” rather than having firm scientific evidence.
In the early days of melissopalynology, during the mid-twentieth century, scientists such as Louveaux, Maurizio, and Vorwohl tried to develop ways of classifying unifloral honey types. Their purpose was to help beekeepers market some of the premium honey types and exclude honey that did not meet those criteria. They suggested that for a honey to be a unifloral, it must contain 45% of a single pollen type. Since those early suggestions, we now know that for some types of unifloral honey that amount of pollen is unreasonable. During the last 50 years, other studies and the development of PC values confirm that for some plant species 45% pollen in honey cannot be reached. Several prime examples include fireweed and sourwood honey, which have highly reduced amounts of pollen in honey yet often they are unifloral types.
A final important aspect about examining pollen in honey is to determine the pollen concentration value. Many melissopalynologist know about how much pollen should occur in certain types of honey. Pollen concentration amounts vary among unifloral honey types and in other types such as multifloral or wildflower honey. Those concentration values can also suggest, but cannot confirm, if honey is adulterated with added sugar. Concentration values can also indicate if a honey is filtered or blended. We have discovered that certain types of commercial honey contain no pollen and thus we cannot determine the origin of the honey or the floral types listed on the labels. For example, we have examined commercial honey purported to be “local” yet it contains no pollen and thus we are unable to confirm if it is indeed local.
For those of you who wish to have your honey analyzed I suggest that you do not filter it at all. Our published results show that almost any type of filtering removes some of the pollen. If various amounts of pollen are inadvertently removed by filtering, the resulting analysis may not be accurate. You can find information about filtering honey in past issues of BEE CULTURE. If you want more information on pollen coefficients just send me an email (vbryant@tamu.edu).









